By Kemo Cham
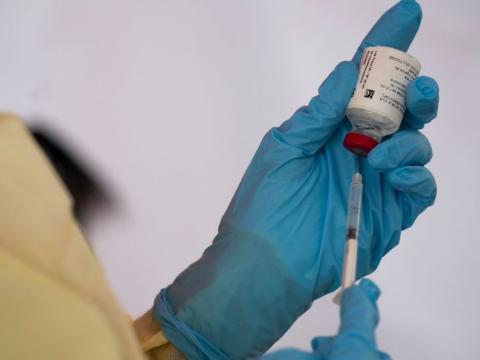
After more than two decades of waiting, the world has finally got a vaccine for the deadly Ebola Virus Disease (EVD).
The European Commission granted marketing authorization to Ervebo, the vaccine created by the pharmaceutical giant, Merck, last Monday. A day later, the World Health Organization (WHO) also announced its pre-qualification approval.
Ervebo also got a node from the European Medicines Agency last month.
Ebola is a viral hemorrhagic fever disease that’s contracted via infected animals and humans. The disease is zoonotic, meaning it can be transmitted from animals to humans.
The disease is highly fatal, as was illustrated by the 2014-2016 West African Ebola Epidemic that ravaged Sierra Leone and its neighbours Liberia and Guinea, infecting nearly 30, 000 people, over 11, 000 of whom died.
Work on Ebola vaccine had been on for decades. The West African outbreak accelerated the search.
Merck, which is an American multinational pharmaceutical company and one of the largest pharmaceutical companies in the world, has been working on the vaccine intensely since the 2014 outbreak.
Ervebo is currently being used in the Democratic Republic of the Congo, which has been battling an Ebola epidemic since August 2018.
“The European Commission’s marketing authorization of Ervebo is the result of an unprecedented collaboration for which the entire world should be proud,” Ken Frazier, Merck’s chairman and chief executive officer, said in a statement released after the approval.
“It is a historic milestone and a testament to the power of science, innovation and public-private partnership,” Frazier added, noting that the company would work with the Food and Drug Administration (FDA) in the United States and regulatory agencies in a number of African countries to license the vaccine.
Approval by the FDA is crucial for the success of the product.
But for it to get a global appeal, approval by WHO, through its vaccine prequalification process, is also important to assure member countries, especially in the developing world, that they are safe and effective.
Prequalification means that the vaccine meets WHO standards for quality, safety and efficacy, and United Nations agencies and Gavi, the Vaccine Alliance, can also procure the vaccine for at-risk countries like Sierra Leone.
“This is a historic step towards ensuring the people who most need it are able to access this life-saving vaccine," WHO Director-General, Dr Tedros Adhanom Ghebreyesus, said in a statement following announcement of the prequalification approval.
"Five years ago, we had no vaccine and no therapeutics for Ebola. With a prequalified vaccine and experimental therapeutics, Ebola is now preventable and treatable," he added.
Prior to the WHO’s decision, Dr Ghebreyesus was quoted describing news about the EU approval as a “great news”.
“This is great news that will change Ebola prevention in the future and protect the vulnerable,” he said.
Copyright © 2019 Politico Online